Abstract
Background:
Mantle cell lymphoma (MCL) is still an incurable disease. While OS has significantly improved in recent years with more effective treatments, early relapse/refractoriness to induction treatment is associated with a dismal outcome (Visco, BJH 2019; Eskelund, HemaSphere 2021). Early identification of patients with MCL who will relapse early or not respond after induction could improve their management.
Patients and methods:
We retrospectively reviewed the characteristics of all consecutive patients diagnosed with MCL in our center from April 2000 to January 2021. The analysis included OS, PFS, DOR, age group (≤65 or >65 years old), morphology (classic vs pleomorphic/blastoid), Ki67 (<30% or ≥30%), MIPI, type of induction: HiDAC-based (high-dose citarabine-based) or non-HiDAC regimens, and early versus late relapse (defined as POD<24 or POD>24 when the relapse occurred ≤24 months or >24 months from diagnosis to the start date of the 1 st salvage treatment). Other factors currently known as relevant (SOX11, TP53 mutational status, karyotype, IGVH status, or other mutations) could not be evaluated due to the low number of patients with available data.
Statistical analyses were performed with the R-4.1.0 software: Kaplan-Meier estimator for OS, PFS, DOR and median survival times, Cox regression for univariate and multivariate analyses and HR calculation, Log-rank test for comparison of OS, PFS, DOR between risk groups, and Fisher's exact test for the analysis of factors affecting POD24.
Results:
One hundred and twenty-five patients [Male:Female 91(72.8%):34(27.2%)] with MCL were analyzed. Age at diagnosis was ≤65 years in 60 patients (48%) and >65 years in 65 (52%), stage III-IV in 92.8%, MIPI was intermediate and high-risk in 22.6% and 53.6% respectively, Ki67 was ≥30% in 40 patients (64.5%) and MCL morphology was pleomorphic/blastoid in 40 patients (13.6%).
Among 123 patients receiving at least 2 treatment lines (induction and 1 st salvage treatment), 36 (29.2%) received HiDAC and 87 (70.7%) a non-HiDAC induction. Among the 73 patients requiring salvage treatment, 45 (61.6%) were POD<24 and 28 (38.3%) were POD>24.
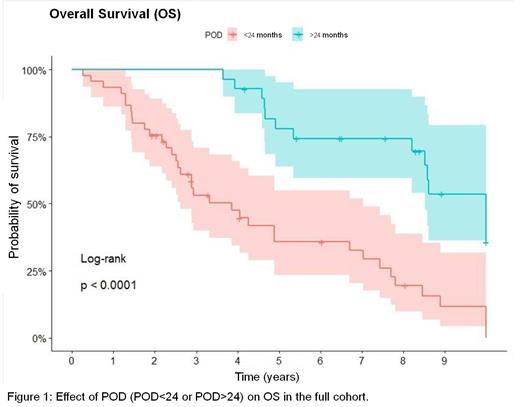
At last follow-up (FU), 50 pts were alive (40%), 7 were lost to FU (5.6%) and 68 had died (54.4%), mainly due to lymphoma-related causes (69.1%). Median OS and PFS were 7.44 and 2.52 years respectively, and median DOR was 34.9 months. There were significant differences on outcome by age group, OS was 10 vs 4.89 years (p=.0021), PFS 4.11 vs 1.64 years (p=.00012) and DOR 57 vs 15.5 months (p=.014) for patients with ≤65 and >65 years, respectively. Regarding other prognostic factors, MIPI did not impact OS, PFS nor DOR. Ki67, blastoid variant and POD24 had impact on OS in the univariate analysis, and only POD<24 maintained its significant effect on OS in the multivariate analysis (p=0.05). Of note, POD24 affected OS in the full cohort (3.86 and 10 years in POD<24 and POD>24, respectively (p=.0001) (Figure 1)) and by age group (3.32 years in POD<24 and 10 years in POD>24 for ≤65 years (p=.00057) and 3.86 in POD<24 and 8.58 years in POD>24 for patients >65 years (p=.0037)).
Finally, we analyzed factors potentially affecting POD24, and we found significant differences with the age group and type of induction.In patients >65 years, 75.6% relapsed early (POD<24), while in the younger group only 43.7% were POD<24 (p=.0076). Regarding the type of induction, in the HIDAC group only 33.3% were POD<24, while 68.9% were POD<24 in the non-HiDAC group (p=.017).
Conclusions:
In our cohort, POD<24 is a strong prognostic factor affecting outcome, with OS of 10 years in POD>24 vs 3.86 years in POD<24. Although younger patients receiving HiDAC-based induction were less likely to be POD<24 and had a significantly better outcome, a subgroup of them still relapse early or do not respond to induction, and for these higher-risk patients, newer and more effective treatment strategies are very much needed.
Abrisqueta: AstraZeneca: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Bobillo: F. Hoffmann-La Roche Ltd: Consultancy, Speakers Bureau; Gilead: Speakers Bureau. Carpio: Regeneron, TAKEDA, Celgene, Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other. Iacoboni: BMS/Celgene, Gilead, Novartis, Janssen, Roche: Honoraria. Gironella Mesa: BMS, Janssen: Honoraria. Palacio: Bristol Myers Squibb (BMS): Consultancy. Bosch: Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Other: Travel. Marin-Niebla: Kiowa Kirin: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel; Roche: Honoraria; Takeda: Consultancy, Honoraria, Other: travel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal